Introduction: B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR T) therapy has revolutionized the treatment for relapsed/refractory multiple myeloma (RRMM). Two BCMA-directed CAR T products, Idecabtagene Vicleucel (ide-cel) and Ciltacabtagene Autoleucel (cilta-cel), have received approval from the U.S. Food and Drug Administration (FDA). While these therapies have demonstrated remarkable efficacy, concerns regarding treatment-related neurotoxicities have emerged. The specific adverse effect of Parkinsonism associated with BCMA-directed CAR T therapy has not been adequately studied.
Methods: We conducted an analysis utilizing the FDA Adverse Events Reports System (FAERS) database, covering the years 2021-2023. Our search focused on the reported incidence of Parkinsonism associated with BCMA-directed CAR T therapy.
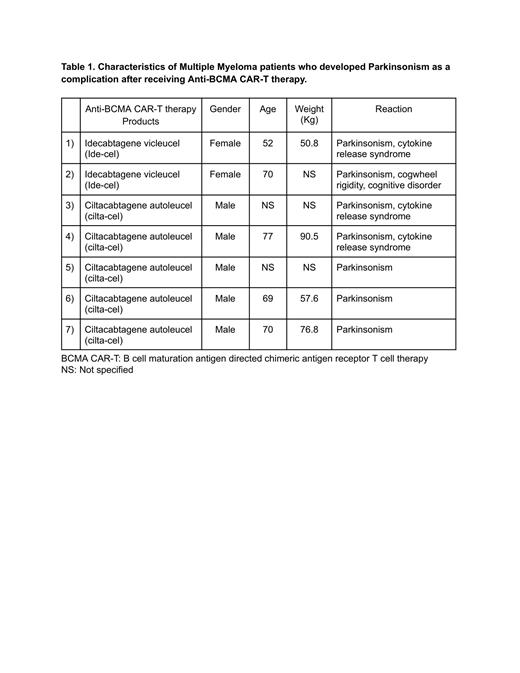
Results: Upon reviewing the FDA data, we identified a total of 177 patients with reported nervous system disorders related to Ide-cel therapy. Out of these patients, 2 had reported incidents of Parkinsonism. Similarly, for Cilta-cel therapy, there were 81 patients with reported nervous system disorders, with 5 reported incidents of Parkinsonism. Out of the total 7 reported patients with Parkinsonism, 5 were males, 2 were females, the average age was 67, the average weight was 68.9 kg, and 3 patients were also noted to have associated cytokine release syndrome.
Conclusion: Our analysis of FAERS data highlights the occurrence of Parkinsonism associated with BCMA-directed CAR T-cell therapy. Further studies are required to quantify the need for neurological surveillance after initiating treatment with these novel agents.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal